PRODUCT DETAIL
SPECIMEN PREPARATION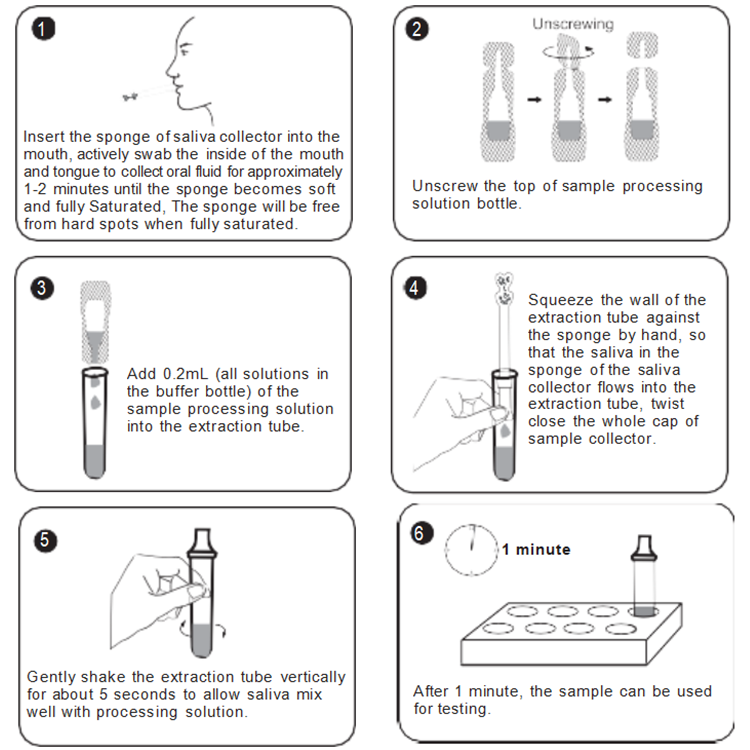
Note:
1. Under normal temperature, samples should be processed within 1 hour after collection. If the sample cannot be tested in time after treatment, the processed sample should be refrigerated at 2-8 ℃ and the test should be completed within 12 hours after treatment.
2. Freezing and thawing of samples is strictly prohibited.
TEST PROCEDURE

STORAGE AND STABILITY
Storage: store at 2~30℃. Shelf life: 24 months.
PERFORMANCE CHARACTERISTICS
1. Analytical Sensitivity
The Coretests® COVID-19 Saliva Ag Test LOD was confirmed as 22.5 TCID50/mL.
2. Analytic Specificity
Results demonstrated that Coretests® COVID-19 Saliva Ag Test has no significant cross-reactivity with the seromarkers listed following:
|
Potential Cross-Reactant |
Test Concentration |
|
|
Virus |
Adenovirus |
1.0 x 10⁵ TCID50/ml |
|
Human metapneumovirus (hMPV) |
1.0 x 10⁵ TCID50/ml |
|
|
Rhinovirus |
1.0 x 10⁵ PFU/ml |
|
|
Enterovirus/Coxsackievirus B4 |
1.0 x 10⁵ TCID50/ml |
|
|
Human coronavirus OC43 |
1.0 x 10⁵ TCID50/ml |
|
|
Human coronavirus 229E |
1.0 x 10⁵ TCID50/ml |
|
|
Human coronavirus NL63 |
1.0 x 10⁵ TCID50/ml |
|
|
Human parainfluenza virus 1 |
1.0 x 10⁵ TCID50/ml |
|
|
Human parainfluenza virus 2 |
1.0 x 10⁵ TCID50/ml |
|
|
Human parainfluenza virus 3 |
1.0 x 10⁵ TCID50/ml |
|
|
Human parainfluenza virus 4 |
1.0 x 10⁵ TCID50/ml |
|
|
Influenza A |
1.0 x 10⁵ TCID50/ml |
|
|
Influenza B |
1.0 x 10⁵ TCID50/ml |
|
|
Respiratory Syncytial Virus A |
1.0 x 10⁵ PFU/ml |
|
|
Bacteria |
Bordetella pertussis |
1.0 x 10⁶ cells/ml |
|
Chlamydia pneumoniae |
1.0 x 10⁶ IFU/ml |
|
|
Haemophilus influenzae |
1.0 x 10⁶ cells/ml |
|
|
Legionella pnuemophila |
1.0 x 10⁶ cells/ml |
|
|
Mycoplasma pneumoniae |
1.0 x 10⁶ U/ml |
|
|
Streptococcus pneumoniae |
1.0 x 10⁶ cells/ml |
|
|
Streptococcus pyogenes (group A) |
1.0 x 10⁶ cells/ml |
|
|
Mycobacterium tuberculosis |
1.0 x 10⁶ cells/ml |
|
|
Staphylococcus aureus |
1.0 x 10⁶ org/ml |
|
|
Staphylococcus epidermidis |
1.0 x 10⁶ org/ml |
|
|
Pooled human nasal wash |
N/A |
|
|
Yeast |
Candida albicans |
1.0 x 10⁶ cells/ml |
1. Interference
The following substances and conditions were found not to interfere with the test. List of potentially interfering compounds and concentrations tested are as follows:
|
Substance |
Active Ingredient |
Concentration |
|
Endogenous |
Mucin |
2% w/v |
|
Whole Blood |
1% v/v |
|
|
Throat Lozenge |
Benzocaine, Menthol |
0.15% w/v |
|
Sore Throat Phenol Spray |
Phenol |
15% v/v |
|
Anti-viral Drug |
Tamiflu (Oseltamivir Phosphate) |
0.5% w/v |
|
Antibacterial, Systemic |
Tobramycin |
0.0004% w/v |
A clinical study using a total 712 saliva samples was conducted. The results of the COVID-19 Saliva Ag Test were compared with a nucleic acid detection test . The diagnostic sensitivity and specificity of the test results are given as below:
Table 1 - Comparison of COVID-19 Saliva Ag Test Device
|
Reference |
Results of Nucleic acid detection test |
Total Results |
||
|
Positive |
Negative |
|||
|
Results of Coretests test |
Positive |
161 |
5 |
166 |
|
Negative |
4 |
542 |
546 |
|
|
Total Results |
165 |
547 |
712 |
|
Results gave Sensitivity is 97.6% (161/165), specificity is 99.1% (542/547), and a total agreement of 98.7% (703/712).

![[XG001]COVID-19 Saliva Ag Test-HUBEI MEIBAO BIOTECHNOLOGYCO., LTD](https://img-va.myshopline.com/image/store/2000919872/1651046960543/7af6d27fc6a44e5ca2f7ace8e0ed9009.png?w=800&h=800)
![[XG001]COVID-19 Saliva Ag Test-HUBEI MEIBAO BIOTECHNOLOGYCO., LTD](https://img-va.myshopline.com/image/store/2000919872/1651046960543/5c9fe6f2ce2c4fd1978f1fcc17ca2a91.png?w=800&h=800)
![[XG001]COVID-19 Saliva Ag Test-HUBEI MEIBAO BIOTECHNOLOGYCO., LTD](https://img-va.myshopline.com/image/store/2000919872/1651046960543/b877392aacdc41098229a48c63881fba.png?w=800&h=800)
![[XG001]COVID-19 Saliva Ag Test-HUBEI MEIBAO BIOTECHNOLOGYCO., LTD](https://img-va.myshopline.com/image/store/2000919872/1651046960543/c243a42624ee4308bd2d27db513be753.png?w=800&h=800)
![[XG001]COVID-19 Saliva Ag Test-HUBEI MEIBAO BIOTECHNOLOGYCO., LTD](https://img-va.myshopline.com/image/store/2000919872/1651046960543/16da8ad84a074e33905a0dd65857cca4.png?w=800&h=800)
![[XG001]COVID-19 Saliva Ag Test-HUBEI MEIBAO BIOTECHNOLOGYCO., LTD](https://img-va.myshopline.com/image/store/2000919872/1651046960543/7755586f356a49c3947bf41b58a28f5e.png?w=800&h=800)


