PRODUCT DETAIL
REAGENT PREPARATION AND STORAGE INSTRUCTIONS
All reagents are ready to use as supplied. Store unused test devices unopened at 2°C-30°C. The positive and negative controls should be kept at 2°C-8°C. If stored at 2°C-8°C, ensure that the test device is brought to room temperature before opening. The test device is stable through the expiration date printed on the sealed pouch. Do not freeze the kit or expose the kit over 30°C.
SPECIMEN COLLECTION AND HANDLING
Consider any materials of human origin as infectious and handle them using standard biosafety procedures.
Plasma
- Collect blood specimen into a lavender, blue or green top collection tube (containing EDTA, citrate or heparin, respectively in Vacutainer® ) by veinpuncture.
- Separate the plasma by centrifugation.
- Carefully withdraw the plasma into new pre-labeled tube.
Serum
- Collect blood specimen into a red top collection tube (containing no anticoagulants in Vacutainer®) by veinpuncture.
- Allow the blood to clot.
- Separate the serum by centrifugation.
- Carefully withdraw the serum into a new pre-labeled tube.
Test specimens as soon as possible after collecting. Store specimens at 2°C-8°C if not tested immediately.
Store specimens at 2°C-8°C up to 5 days. The specimens should be frozen at -20°C for longer storage.
Avoid multiple freeze-thaw cycles. Prior to testing, bring frozen specimens to room temperature slowly and mix gently. Specimens containing visible particulate matter should be clarified by centrifugation before testing.
Do not use samples demonstrating gross lipemia, gross hemolysis or turbidity in order to avoid interference on result interpretation.
Blood
Drops of whole blood can be obtained by either finger tip puncture or veinpuncture. Do not use any hemolized blood for testing.
Whole blood specimens should be stored in refrigeration (2°C-8°C) if not tested immediately. The specimens must be tested within 24 hours of collection.
ASSAY PROCEDURE
(2) Drop 3 drops of diluted specimen vertically into the specimen well (S) of the test cassette avoiding the formation of bubbles.
(3) Observe the test results immediately within 15-20 minutes, the result is invalid over 25 minutes.
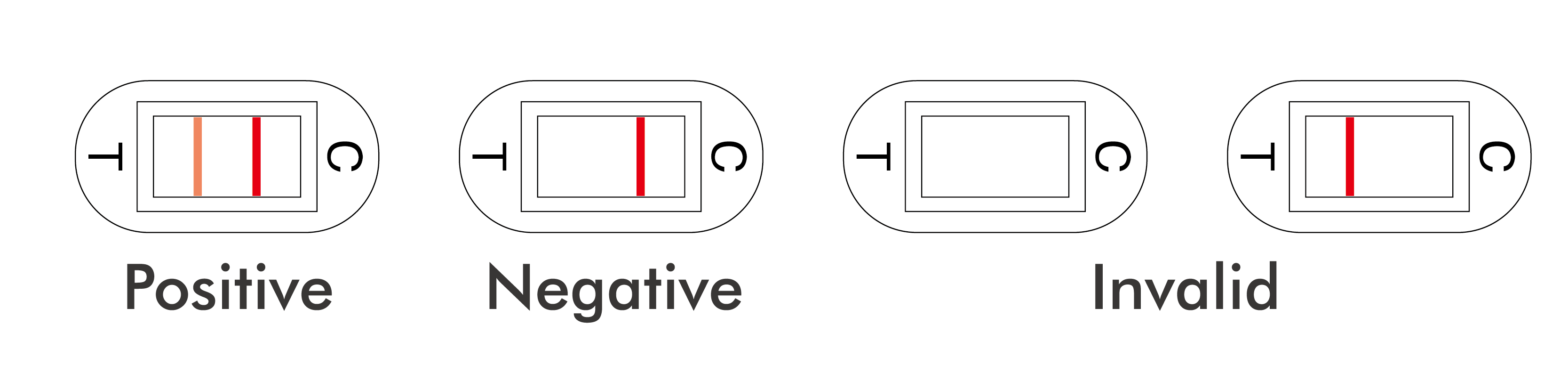
A pink to red line (T), even if it is very thin, indicates a positive result.
NEGATIVE: A red line appears in the control region(C). No line appears in the test region (T).
INVALID: No red lines appear or control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the kit and contact your local distributor.